FDA approves Orthofix's ultrasonic bone fracture healing system
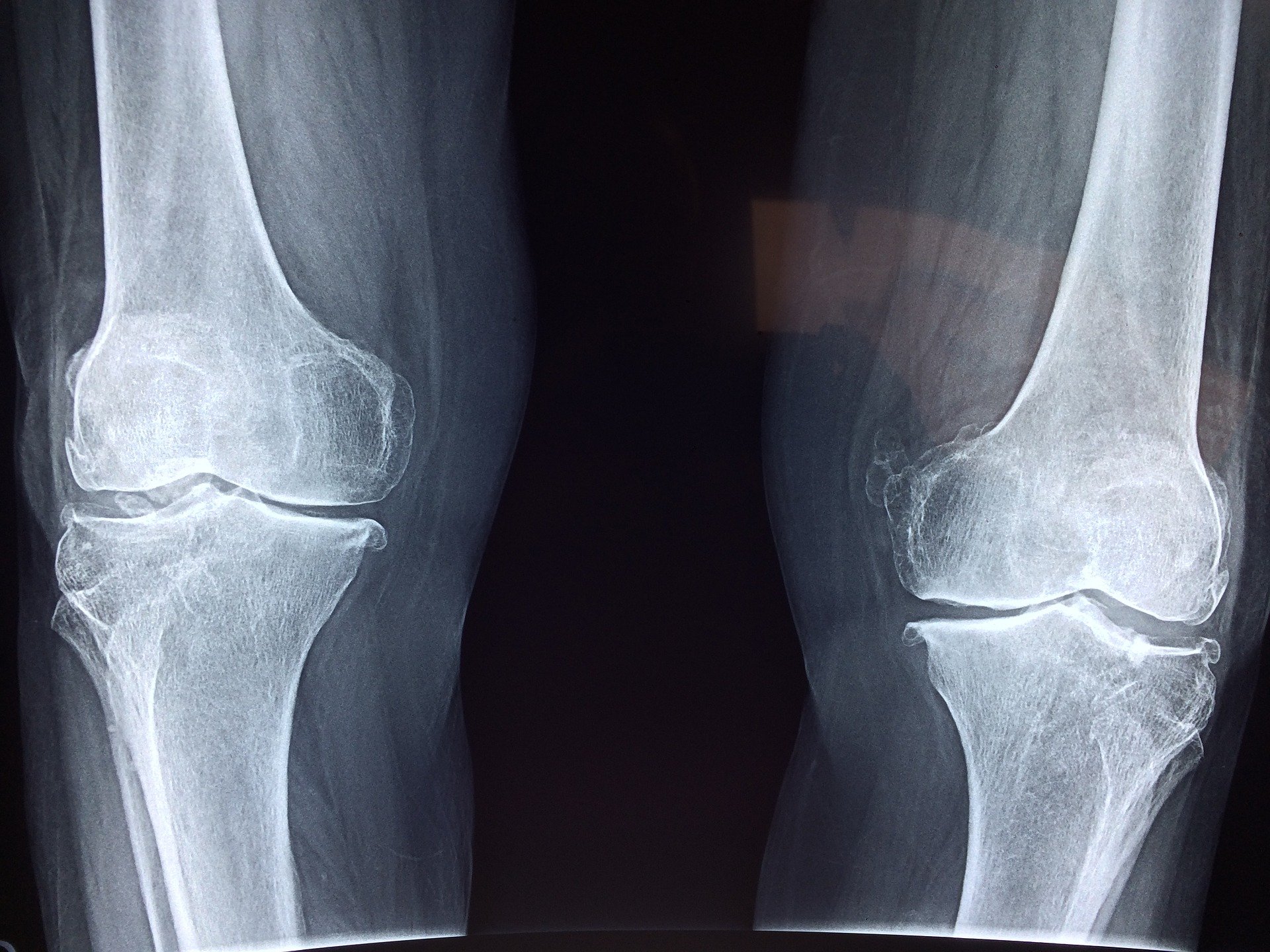
FDA approves Orthofix's ultrasonic bone fracture healing system
Sporting autographed casts for months may one day become a thing of the past. | The AccelStim system is a nonsurgical treatment designed to be worn for 20 minutes per day for fresh bone breaks as well as fractures that have not healed on their own.
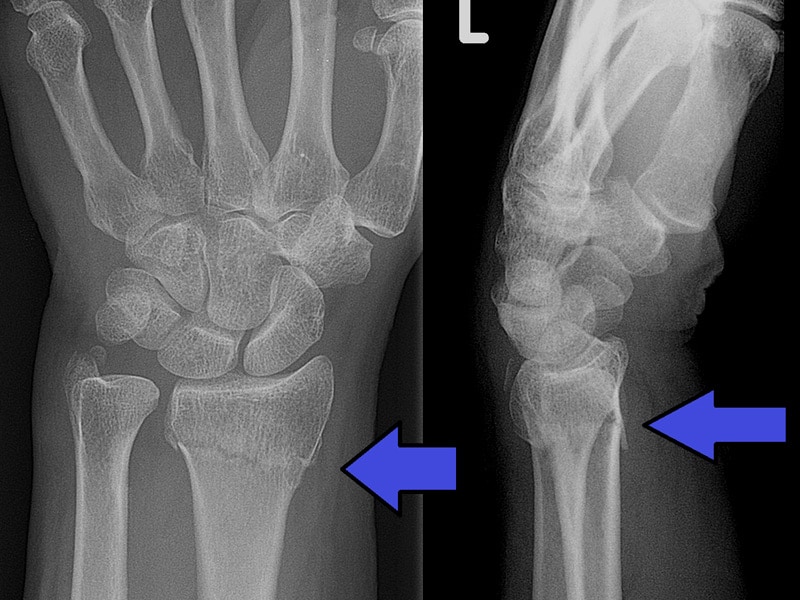
FDA Approves AI-Based Software for Wrist Fracture Detection

The Orthofix Philosophy - Orthofix

Orthofix LinkedIn

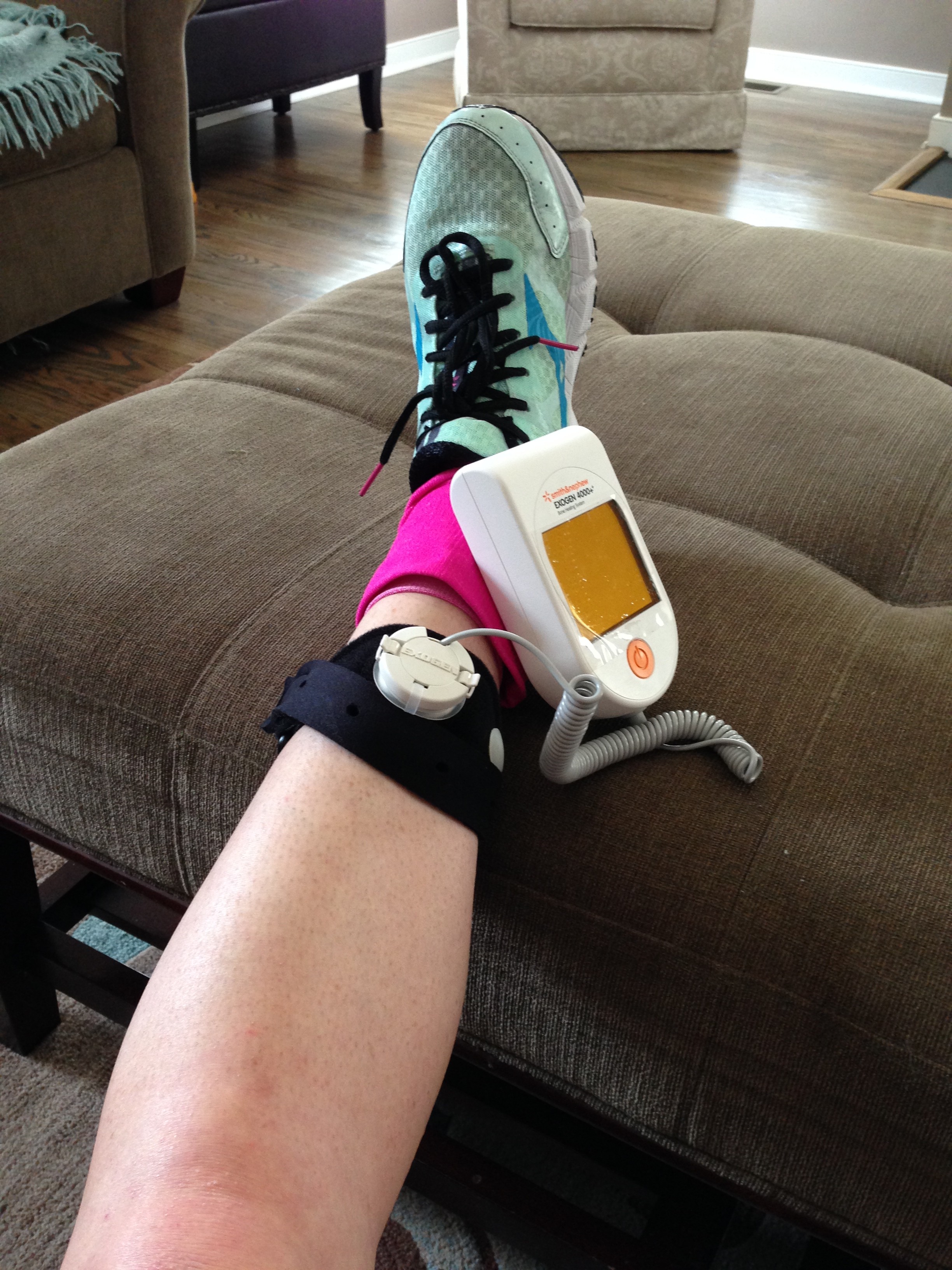
Bone Growth Stimulator Market Size & Share Analysis - Industry
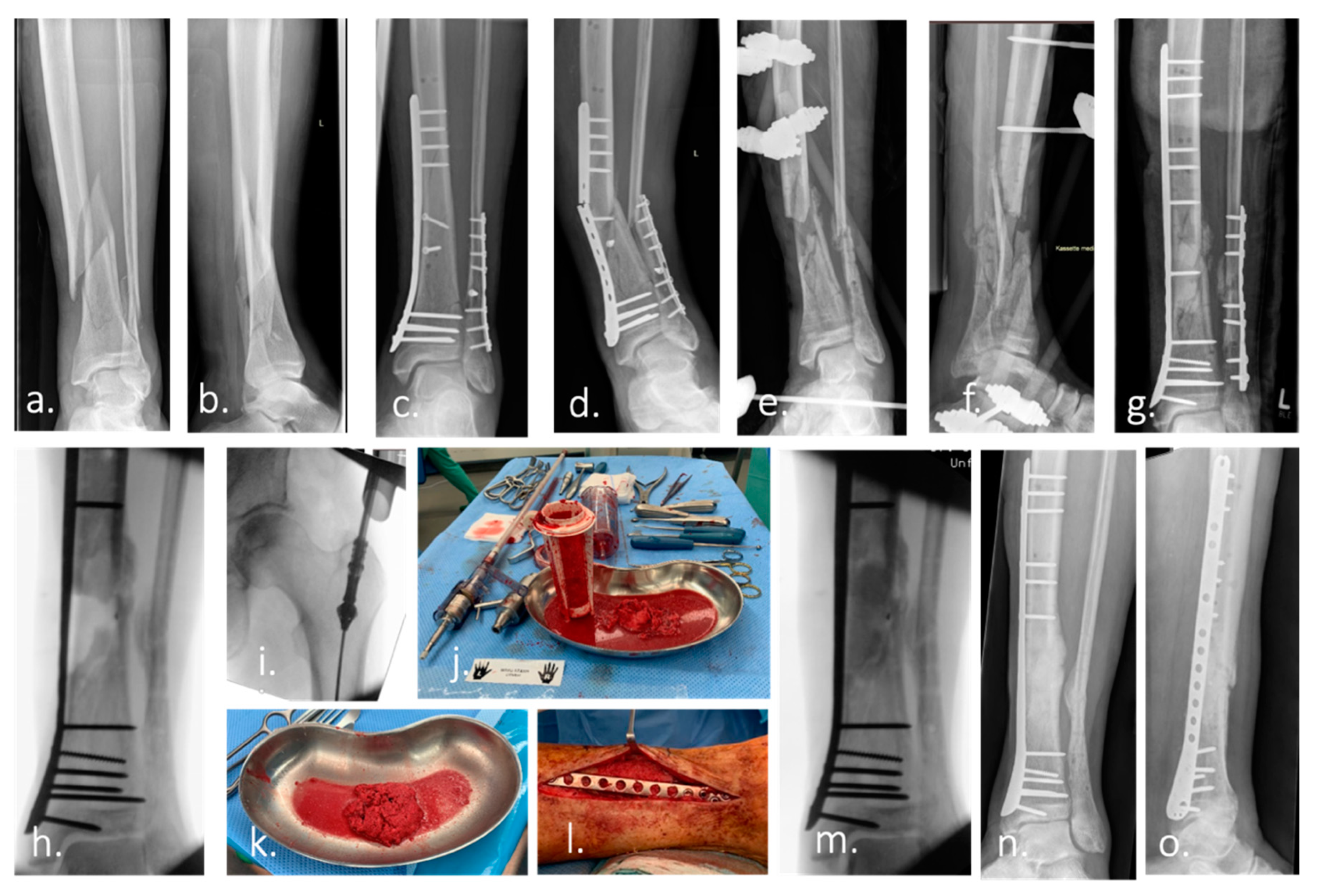
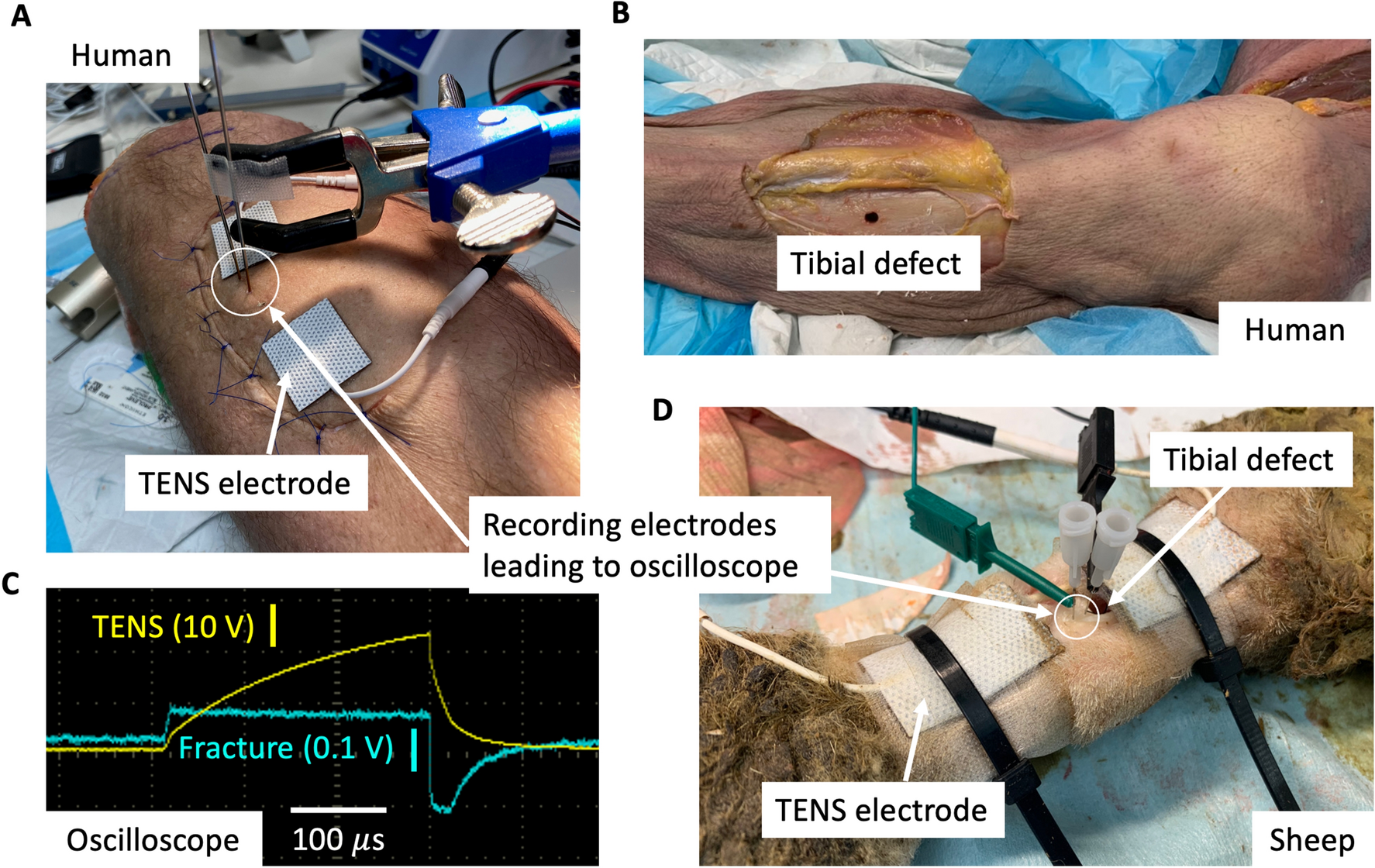
IJMS, Free Full-Text

A depiction of inductive coupling stimulation for treatment of a

FDA Grants PMA for the AccelStim Bone Growth Stimulation Device
Device for Treating Challenging Bone Fractures Cleared by FDA

Orthofix Launches the Galaxy Fixation Gemini System in the U.S.
:max_bytes(150000):strip_icc()/GettyImages-568775841-56a516695f9b58b7d0dac8b6.jpg)